Chronic Myeloid Leukemia Overview
by Dr. Joseph Barone, BCOP
Background
Chronic Myeloid Leukemia (CML) is a slowly progressing cancer of the blood and bone marrow, which is characterized by the presence of the Philadelphia chromosome (Ph+). Ph+ results from a genetic mutation due to a translocation between chromosomes 9 and 22 [t(9;22)], which leads to the presence of an abnormal fusion gene, known as BCR-ABL1. This fusion gene alters multiple signaling pathways within cells that directly affect apoptotic potential, cell division rates, and different stages of the cell cycle to achieve unabated white blood cell proliferative potential.1
CML comprises 15% of all adult leukemia diagnoses.2 It is estimated that 8,450 patients will be diagnosed with CML in 2020 while an estimated 1,130 CML patient deaths are expected in 2020. The median age at initial CML diagnosis is 65 years old. The median age at death for a patient with CML is 77 years old. CML has a median five-year overall survival of 70.4%.3
Diagnosis/Staging
Initial evaluation for a patient suspected of having CML includes a history and physical exam (including splenic palpation) and complete blood count (CBC) with differential, chemistry panel, and hepatitis panel. Additionally, a bone marrow aspirate and biopsy for morphologic and cytogenetic evaluation and quantitative reverse transcriptase-polymerase chain reaction (qPCR) using International Scale (IS)to establish the presence of quantifiable BCR-ABL1 mRNA transcripts at baseline are recommended to confirm the diagnosis of CML.1
CML occurs in three distinct phases (chronic, accelerated, and blast) and is usually diagnosed in the chronic phase. The initial evaluation places the patient’s CML into one of three phases, which guides treatment decisions. Chronic phase CML includes patients with peripheral blood myeloblasts less than 15% with no other criteria of accelerated or blast phase CML. According to the Modified MD Anderson Cancer Center (MDACC), accelerated phase CML includes patients with: (1) peripheral blood myeloblasts less than 30% but greater than or equal to 15%, (2) peripheral blood myeloblasts and promyelocytes combined greater than or equal to 30%, (3) peripheral blood basophils greater than or equal to 20%, (4) platelet count less than or equal to 100 x 109/L unrelated to therapy, and (5) additional clonal cytogenetic abnormalities in Ph+ cells. The International Bone Marrow Transplant Registry defines blast phase CML as CML with greater than or equal to 30% myeloblasts in the blood, bone marrow, or both, or the presence of extramedullary infiltrates of leukemic cells.1
Treatment of Chronic Phase/Accelerated Phase CML
Treatment considerations for chronic phase CML include comorbidities, toxicity profile of tyrosine kinase inhibitors (TKIs), possible drug interactions, patient preference, and CML risk score. The figure below displays the various scoring methodologies used to assess a patient’s CML risk score. Scoring systems incorporate patient age, spleen size, and complete blood count with differential results.
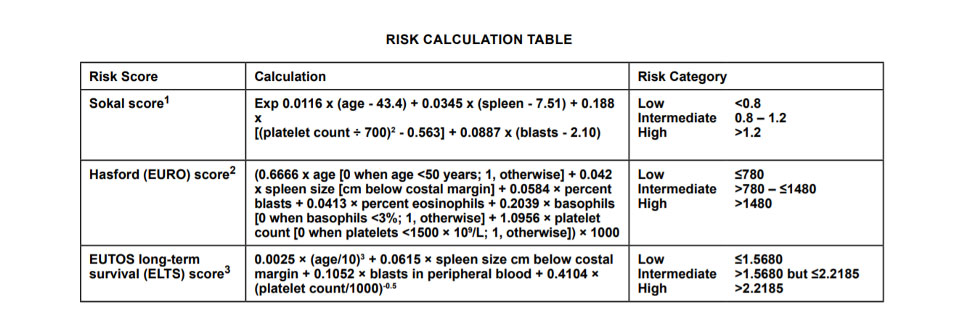
For patients with chronic phase CML and a low-risk score, preferred regimens include imatinib 400 mg daily, bosutinib 400 mg daily, dasatinib 100 mg daily, nilotinib 300 mg twice daily, or a clinical trial. For patients with chronic phase CML and an intermediate or high-risk score, preferred regimens include bosutinib 400 mg daily, dasatinib 100 mg daily, or nilotinib 300 mg twice daily. Other recommended regimens for chronic phase CML patients with an intermediate or high-risk score include imatinib 400 mg daily or a clinical trial.1
Preferred regimens for accelerated phase CML include either a clinical trial, bosutinib, dasatinib, nilotinib, or ponatinib. Other recommended regimens include imatinib or in certain circumstances omacetaxine.1
Response to treatment is evaluated in relation to criteria for hematologic, cytogenetic, and molecular responses as well as relapse. A complete hematologic response occurs with the following: (1) complete normalization of peripheral blood counts with leukocyte count < 10 x 109/L, (2) platelet count < 450 x 109/L, (3) no immature cells, such as myelocytes, promyelocytes, or blasts in peripheral blood, and (4) no signs and symptoms of disease with a resolution of palpable splenomegaly. A complete cytogenetic response (CCyR) occurs with there are no Ph-positive metaphases. A major molecular response (MMR) occurs when BCR-ABL1 (IS) is ≤ 0.1% by qPCR or there is ≥ a 3-log reduction in BCR-ABL1 mRNA from the standardized baseline if qPCR (IS) is not available.1
In evaluating response to TKI treatment, CML disease may be categorized as TKI-sensitive, TKI-resistant, or possible TKI-resistant. With TKI-sensitive disease, BCR-ABL1 (IS) is ≤ 1% by qPCR for at least the first 15 months of treatment or >1%-10% for the first six months of treatment and the same TKI should be continued. With TKI-resistant disease, BCR-ABL1 (IS) is > 10% by qPCR after three months of treatment or > 1%-10% after 12 months of treatment and an alternate TKI should be started and the patient should be evaluated for allogeneic hematopoietic cell transplant (HCT). With possible TKI resistance, BCR-ABL1(IS) is > 10% by qPCR in the first three months of treatment or > 1%-10% after six months of treatment and the TKI should be switched and the patient should be considered for allogeneic HCT. If imatinib was used as first-line therapy, the dose should be increased to 800 mg daily. With both TKI-resistant disease and possible TKI resistance, patient compliance and drug interactions should be evaluated. Additionally, for CML patients who are unable to achieve MMR, BCR-ABL1 kinase domain mutation testing should be performed as this assay may identify potential drug-resistant mutations.1
Discontinuation of TKI Therapy for Chronic Phase CML Patients
Although CML therapy with a TKI was previously given for the duration of the patient’s lifespan, clinical trials have been performed to identify chronic phase CML patients who are appropriate candidates for therapy discontinuation depending on certain conditions. According to the NCCN guidelines for CML, all of the following criteria must be met for a patient to be considered for therapy discontinuation: 1) age >18 years old, 2) chronic phase CML only (no prior history of accelerated phase or blast phase CML), 3) has received TKI therapy for at least three years, 4) prior evidence of BCR-ABL1 transcript by qPCR, 5) stable molecular response (i.e. BCR-ABL1 (IS) < 0.01% by qPCR) for at least two years as documented on at least four tests performed at least three months apart, 6) access to reliable qPCR test with a sensitivity of at least BCR-ABL1(IS) < 0.0032% by qPCR and that provides results within two weeks, 7) monthly molecular monitoring for one year, then every two months for the second year, and every three months thereafter. TKI therapy should be resumed within four weeks of any loss of MMR.
Treatment of Blast Phase CML
Prior to treatment of blast phase CML, it must be determined if the blast phase involves myeloid or lymphoid blast cell lineage. With myeloid blast phase, treatment may include a clinical trial, acute myeloid leukemia (AML)-type induction chemotherapy plus a TKI or monotherapy with a TKI. With lymphoid blast phase, treatment may include a clinical trial, acute lymphoblastic leukemia (ALL)-type induction chemotherapy plus a TKI, or a TKI plus corticosteroids. Patients would be evaluated for an allogeneic HCT regardless of blast cell lineage.1
Medications Used in the Treatment of CML
Gleevec® (Imatinib)
Imatinib is indicated for newly diagnosed adult and pediatric patients with Ph+ CML in the chronic phase and in patients with Ph+ CML in blast crisis, accelerated phase, or in chronic phase after failure of prior interferon-alpha therapy. The adult dose for chronic phase CML is 400 mg daily but may be increased to 600 mg daily. The adult dose for the accelerated phase or blast phase is 600 mg daily but may be increased to 400 mg twice daily. The pediatric dose for Imatinib is 340 mg/m2 (not to exceed 600 mg) orally once daily or divided twice daily. The starting dose may be reduced for moderate renal impairment (CrCl 20-39 mL/min) by 50% and should be used in caution in patients with severe impairment (CrCl <20 mL/min). The starting dose should be reduced by 25% in patients with severe hepatic impairment (i.e. Child Pugh C). Imatinib is a major substrate and a moderate inhibitor of CYP3A4. Imatinib is a minor substrate of CYP1A2, CYP2C19, CYP2C8, CYP2C9, and CYP2D6. Imatinib is also a substrate of P-glycoprotein/ABCB1. Further information regarding Imatinib drug interactions may be found in the Appendix.
Imatinib has the following warnings or precautions: bone marrow suppression, cardiovascular effects including severe heart failure and left ventricular dysfunction, dermatologic reactions, hypothyroidism, impairments related to driving and using machinery, fluid retention/edema, gastrointestinal toxicity, hemorrhage, hepatotoxicity, nephrotoxicity, growth retardation in children and adolescents, embryo-fetal toxicity, and tumor lysis syndrome. The most frequently reported adverse drug reactions (occurring in > 30% of patients) include edema, nausea, vomiting, muscle cramps, musculoskeletal pain, diarrhea, rash, fatigue, and abdominal pain.
Imatinib tablets should be administered with a meal and a large glass of water. Tablets should not be crushed. Tablets may be dispersed in water or apple juice (using ~50 mL for 100 mg tablet, ~200 mL for 400 mg tablet); stir until dissolved and administer immediately. Grapefruit juice should be avoided. Imatinib tablets are available as 100 mg and 400 mg strengths and should be stored at room temperature.4,5
Tasigna® (Nilotinib)
Nilotinib is indicated for adult and pediatric patients greater than or equal to 1 year of age with newly diagnosed Ph+ CML in chronic phase, adult patients with chronic phase or accelerated phase Ph+ CML resistant to or intolerant to prior therapy that included Imatinib, and pediatric patients greater than or equal to 1 year of age with Ph+ CML chronic phase resistant or intolerant to prior tyrosine kinase inhibitor therapy. The adult dose for newly diagnosed chronic phase CML is 300 mg twice daily. The adult dose for resistant or intolerant accelerated phase or chronic phase CML is 400 mg twice daily. The pediatric dose for newly diagnosed or previously treated patients is 230 mg/m2 orally twice daily, rounded to the nearest 50 mg dose (to a maximum single dose of 400 mg). There are no dosage adjustments provided in the manufacturer’s labeling for renal dysfunction. For patients with newly diagnosed Ph+ CML in chronic phase with hepatic impairment (Child-Pugh class A, B, or C), the initial dose is reduced to 200 mg twice daily. For patients with resistant or intolerant Ph+ CML in chronic or accelerated phase, the initial dose is reduced to 300 mg twice daily with mild to moderate impairment (Child-Pugh class A or B) and 200 mg twice daily with severe impairment (Child-Pugh class C). Nilotinib is a major substrate and a moderate inhibitor of CYP3A4. It is also a substrate of P-glycoprotein/ABCB1. Further information regarding Nilotinib drug interactions may be found in the Appendix.
Nilotinib is contraindicated in patients with hypokalemia, hypomagnesemia, or long QT syndrome. Nilotinib has the following warnings or precautions: bone marrow suppression, cardiac and arterial vascular occlusive events, pancreatitis and elevated serum lipase, hepatotoxicity, electrolyte abnormalities, tumor lysis syndrome, hemorrhage, fluid retention, effects on growth and development in pediatric patients, and embryo-fetal toxicity. Commonly reported non-hematologic adverse drug reactions (occurring in > 20% of adult and pediatric patients) include nausea, rash, headache, fatigue, pruritis, vomiting, diarrhea, cough, constipation, arthralgia, nasopharyngitis, pyrexia, and night sweats. Common hematologic adverse events include thrombocytopenia, neutropenia, anemia.
Nilotinib capsules should be administered twice daily about 12 hours apart on an empty stomach, at least one hour before or two hours after food. The capsules should be swallowed whole with water. If unable to swallow whole, the patient may empty the capsule’s contents into 5 mL applesauce (puréed apple) and administer within 15 minutes (do not save for later use). Nilotinib capsules are available in 50 mg, 150mg, and 200 mg strengths and should be stored at room temperature.4,6
Sprycel® (Dasatinib)
Dasatinib is indicated for newly diagnosed adults with Ph+ CML in chronic phase, adults with chronic, accelerated or myeloid or lymphoid blast phase Ph+ CML with resistance or intolerance to prior therapy including Imatinib, and pediatric patients 1 year of age and older with Ph+ CML in chronic phase. The dose for Ph+ ALL is 140 mg once daily. The adult dose for newly diagnosed or resistant or intolerant Ph+ CML in the chronic phase is 100 mg once daily. The adult dose for resistant or intolerant Ph+ CML in the accelerated or blast phase is 140 mg once daily. The pediatric dose for Ph+ CML is based upon body weight as follows: 40 mg once daily in patients who weigh 10-19 kg, 60 mg once daily in patients who weigh 20-29 kg, 70 mg once daily in patients who weigh 30-44 kg, and 100 mg once daily in patients who weigh at least 45 kg. There are no dosage adjustments for patients with pre-existing renal or hepatic impairment. Dasatinib is a major substrate of CYP3A4. Further information regarding Dasatinib drug interactions may be found in the Appendix.
Dasatinib has the following warnings or precautions: bone marrow suppression, cardiovascular events, dermatologic toxicity, fluid retention, hemorrhage, pulmonary arterial hypertension, QT prolongation, embryo-fetal toxicity, effects on growth and development in pediatric patients, and tumor lysis syndrome. The most common adverse drug reactions (occurring in > 15% of adult and pediatric patients with CML) include myelosuppression, fluid retention events, diarrhea, headache, skin rash, hemorrhage, dyspnea, fatigue, nausea, and musculoskeletal pain.
Dasatinib tablets should be administered once daily without regard to food. If gastrointestinal upset occurs, the tablets should be taken with a meal. The tablets should be swallowed whole. Proton pump inhibitors and H2 blockers should be avoided. If needed, antacid administration may be considered but the antacid dose must be separated by at least two hours before or two hours after the dasatinib dose. Grapefruit juice should be avoided. Dasatinib tablets are available in the brand name (Sprycel®) only in 20 mg, 50 mg, 70 mg, 80 mg, 100 mg, and 140 mg strengths and they should be stored at room temperature.4, 7
Bosulif® (Bosutinib)
Bosutinib is indicated for newly diagnosed Ph+ CML in chronic phase and chronic, accelerated, or blast phase Ph+ CML with resistance or intolerance to prior therapy. The dose for newly diagnosed Ph+ CML in the chronic phase is 400 mg once daily although doses may be escalated to 600 mg once daily based on therapy response. The dose for resistant or intolerant Ph+ CML is 500 mg once daily although doses may be escalated to 600 mg once daily based on therapy response. For patients with newly diagnosed Ph+ CML, the bosutinib dose is reduced to 300 mg once daily for CrCl 30 to 50 mL/min and to 200 mg once daily for CrCl < 30 ml/min. For patients with resistant or intolerant Ph+ CML, the bosutinib dose is reduced to 400 mg once daily for CrCl 30 to 50 mL/min and to 300 mg once daily for CrCl < 30 mL/min. For patients with preexisting hepatic impairment (Child-Pugh class A, B, or C), the initial bosutinib dose is reduced to 200 mg once daily. Bosutinib is a major CYP3A4 substrate and is known to interact with medications that reduce gastric acid. Further information regarding Bosutinib drug interactions may be found in the Appendix.
Bosutinib has the following warnings: gastrointestinal toxicity, myelosuppression, hepatotoxicity, cardiac failure, fluid retention, nephrotoxicity, and embryo-fetal toxicity. The most common adverse drug reactions (occurring in >20% of patients) include diarrhea, fatigue, nausea, vomiting, anemia, thrombocytopenia, rash, abnormal liver function, pyrexia, cough, headache, edema, and abdominal pain.
Bosutinib tablets should be administered once daily with food. The tablets should be swallowed whole. Bosutinib tablets are available in in the 100 mg, 400 mg, and 500 mg strengths and should be stored at room temperature.4, 8
Iclusig® (Posatinib)
Ponatinib is indicated for patients with T315I-positive CML in the chronic, accelerated, or blast phase and for adult patients with chronic phase, accelerated phase, or blast phase Ph+ CML for whom no other TKI therapy is indicated. The initial dose for both indications is 45 mg once daily. There are no dosage adjustments provided in the manufacturer’s labeling for renal impairment. For patients with mild to severe hepatic impairment (Child-Pugh class A, B, or C), the initial dose should be reduced to 30 mg once daily. Ponatinib is a minor substrate of CYP2D6 and CYP3A4 and it inhibits BSEP/ABCB11. Further information regarding Ponatinib drug interactions may be found in the Appendix.
Ponatinib has the following black box warnings: arterial occlusion, heart failure, hepatotoxicity, and venous thromboembolism. Other warnings or precautions include arrhythmias, bone marrow suppression, fluid retention/edema, GI perforation, hemorrhage, hypertension, neuropathy, ocular toxicity, pancreatitis, reversible posterior leukoencephalopathy (RPLS), tumor lysis syndrome, wound healing impairment, and embryo-fetal toxicity. The most common adverse drug reactions (occurring in >20% of patients) include abdominal pain, rash, constipation, headache, dry skin, arterial occlusion, fatigue, hypertension, pyrexia, arthralgia, nausea, diarrhea, increased lipase, vomiting, myalgia, and extremity pain.
Ponatinib tablets should be administered once daily with or without food. The tablets should be swallowed whole. Grapefruit juice should be avoided. Iclusig tablets are available in in 15 mg and 45 mg strengths and should be stored at room temperature.4, 9
Synribo® (Omacetaxine)
Omacetaxine is indicated for adult patients with CML in the chronic or accelerated phases with resistance or intolerance to two or more TKIs. The dose during induction is 1.25 mg/m2 subcutaneously twice daily for 14 consecutive days of a 28-day cycle and continued until a hematologic response is achieved. The dose during the maintenance phase is 1.25 mg/m2 twice daily for seven consecutive days of a 28-day cycle and continued until no longer achieving clinical benefit. As opposed to Imatinib, Nilotinib, Dasatinib, Bosutinib, and Ponatinib, Omacetaxine is not a BCR-ABL TKI and works via a different mechanism to reduce protein levels of the BCR-ABL oncoprotein as well as Mcl-1, an anti-apoptotic protein, via protein synthesis inhibition. There are no dosage adjustments for renal or hepatic impairment in the manufacturer’s labeling. Omacetaxine is a substrate of P-glycoprotein/ABCB1.
Omacetaxine has warnings or precautions for bone marrow suppression, bleeding, hyperglycemia, and embryo-fetal toxicity. The most common adverse drug reactions associated with Omacetaxine (occurring in >20% of patients) include thrombocytopenia, anemia, neutropenia, diarrhea, nausea, fatigue, asthenia, injection site reaction, pyrexia, infection, and lymphopenia.
Omacetaxine is administered subcutaneously at approximately 12-hour intervals. If home administration is to occur, advise the patient on proper handling, storage conditions, administration, disposal, clean-up of accidental spillage, and ensure that the patient or patient’s caregiver is an appropriate candidate for home administration. Avoid skin and eye contact; wear protective eyewear and gloves during handling and administration. Please refer to full Omacetaxine prescribing information for a detailed procedure on appropriate subcutaneous administration of this medication. Omacetaxine is available as a 3.5 mg vial for reconstitution which should be stored at room temperature prior to use.
Appendix
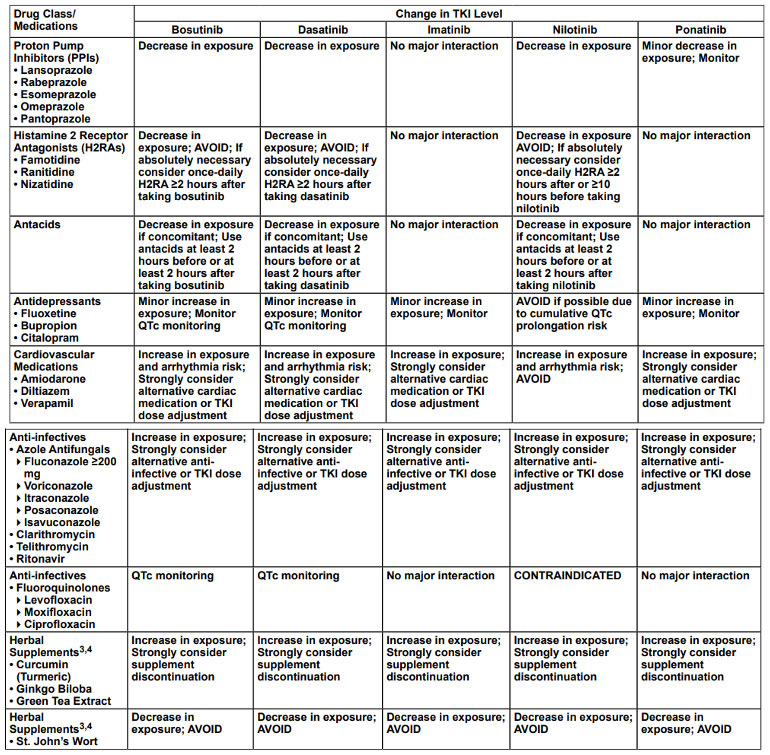
DRUG INTERACTIONS OF THE TKIs WITH MOST COMMONLY USED DRUGS AND SUPPLEMENTS1,2
Drug interactions with TKIs are not uncommon. It is always important to take a detailed medication history (including herbal supplements) at every visit.
References
1 National Comprehensive Cancer Network Chronic Myeloid Leukemia Guidelines Version 3.2020
2 Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2019. CA Cancer J Clin 2019; 69:7-34
3 https://seer.cancer.gov/statfacts/html/cmyl.html
4 LexiComp (online.lexi.com) 2020.
5 https://www.novartis.us/sites/www.novartis.us/files/gleevec_tabs.pdf
6 https://www.novartis.us/sites/www.novartis.us/files/tasigna.pdf
7 https://packageinserts.bms.com/pi/pi_sprycel.pdf
8 http://labeling.pfizer.com/ShowLabeling.aspx?id=884
9 https://www.iclusig.com/pdf/ICLUSIG-Prescribing-Information.pdf
10 https://www.synribohcp.com/wp-content/uploads/synribo_pi.pdf
